Last week I did several Facebook Live “events” about the newest migraine drug Aimovig, a CGRP antagonist released last week from Amgen. (You can see those and other videos here.) There is much to be excited about. Aimovig is the first drug specifically developed for migraines. The other migraine drugs that are used for migraines are actually drugs used for other purposes primarily, but have been found to have a beneficial effect on some migraineurs. Migraine disease is too debilitating and too prevelant to be treated with the secondary effects of a drug. Now Amgen has provided a drug developed for and specifically used for migraines, and they are just the first. By the end of the year we expect similar CGRP antagonist drugs to be released from Lily and others.
How does Aimovig work?
So let’s first discuss… what is CGRP, and why is it important to antagonize it? CGRP stands for calcitonin gene-related peptide, which is a substance that comes from nerve tissue and is felt to be an important mediator of pain processes in the brain. CGRP also acts as a vasodilator, meaning that it causes blood vessels to get larger, and vasodilation has long been felt to be an important cause of pain in migraine disease.
The goal of antagonizing this substance is to prevent the CGRP form doing it’s job- preventing it from causing pain and vasodilation. In order to “antagonize” CGRP, scientists have developed antibodies that bind on to CGRP and prevent it from triggering it’s effects of pain and vasodilation. Antibodies are usually made by a person’s immune system, but in this case scientists have created these blocking molecules in the lab. While the production and mechanism of antibodies is quite complex, you can think of them like the reddish molecules that are binding to the purple CGRP in the graphic above. When the red molecules coat the purple CGRP, the CGRP cannot interact with the receptors in the brain that cause pain and vasodilation. The CGRP is effectively “blocked” or “antagonized” so it cannot perform its function.
So Amgen has created Aimovig as a way for migraine patients to inject these antibodies into their own bodies to allow blockade of the CGRP molecules in their brains. Because these antibodies break down over time, they must be periodically re-injected which is why the drug requires monthly shots. The development of these drugs has taken a long time and a lot of resources, which is one of the reasons that it is so expensive.
Is Aimovig effective?
So we know Aimovig is an exciting new drug that is specifically made to block CGRP and prevent migraine pain. We understand how Aimovig works, but is it effective? The answer is yes, to an extent. The studies that were done on Aimovig showed that it reduced migraines by 1 to 2 1/2 episodes a month.1 The FDA press release which describes the evidence for Aimovig’s effectiveness, called its “efficacy” in scientific circles, can be found here. What is unclear is the number of migraines that the patients had to start with, and therefore the degree or percentage of improvement that should be expected when taking Aimovig.
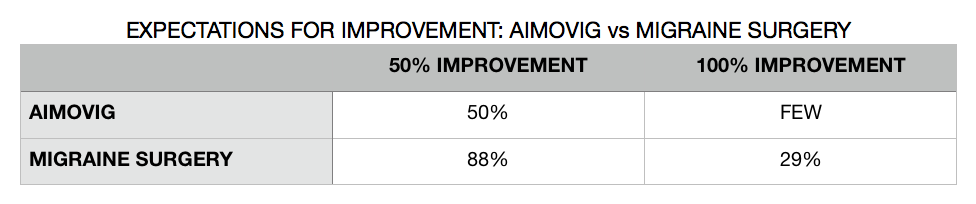
In my discussion with neurologists, the expectations that they have is that Aimvovig should significantly reduce the number of migraines experienced in many patients. The expectation is that about 50% of migraine patients prescribed Aimovig should experience a 50% reduction in their migraines. Said another way, about half of patients will get half as much better than they are without the drug. They expect to see a few “super-responders” as well, in whom Aimovig prevents all further migraines all together.
As a migraine surgeon, I am very excited for this new opportunity for many patients to benefit from a new drug. As many of you know, my attitude toward migraine pain is “do whatever works.” I want patients with migraine and occipital neuralgia to feel better, no matter how that can happen, though I want everyone to avoid narcotics as much as possible. So with that in mind, I am very hopeful that Aimovig is a great addition to the armamentarium of ways neurologists can treat migraine pain.
What is difficult for me to swallow, however, is the following…. Neurologists expect that with Aimovig, 50% of patients will get 50% better, and a small number of patients to get 100% better. With migraine surgery patients, extensive studies have shown that 88% of patients will get at least 50% better, and about 29% of patients will get 100% better.2,3
Migraine treatments and cures: Alternatives to Aimovig
So given the above odds for improvement, would you choose Aimovig or migraine surgery? Let me add a bit to that decision. Aimovig costs are currently approximately $7000 per year, and Aimovig has to be continuously given to provide ongoing results. Over the span of 10 years, treatment with Aimovig will cost about $70,000. Migraine surgery costs are variable, but currently in my practice the cost of migraine surgery ranges between $10,000 and $14000 which is less than or equal to the cost of Aimovig over a 2 year period. And the results are usually permanent, meaning no further therapy nor further expense is required. AND, the results of migraine surgery exceed the expected results with Aimovig.
The thing that Amgen, and therefore Aimovig, does have over migraine surgery in general is money. On the day that Aimovig was widely announced on nearly every media outlet imaginable, 10 children were tragically killed in another deplorable school shooting in Houston. Despite this major news event, Aimovig’s release remained a major topic of discussion on all of the news networks. I was inundated with messages from friends, family, and patients about the article in the New York Times about this new drug Aimovig, and how it is going to be so great for migraines. (Even that article though referenced an editorial in the journal JAMA calling Aimovig “progress but not a panacea.”) The news about the release of Aimovig was everywhere, and as such because of the amazing advertising and media budget that “Big Pharma” such as Amgen has to create buzz and popular demand.So given the above odds for improvement, would you choose Aimovig or migraine surgery? Let me add a bit to that decision. Aimovig costs are currently approximately $7000 per year, and Aimovig has to be continuously given to provide ongoing results. Over the span of 10 years, treatment with Aimovig will cost about $70,000. Migraine surgery costs are variable, but currently in my practice the cost of migraine surgery ranges between $10,000 and $14000 which is less than or equal to the cost of Aimovig over a 2 year period. And the results are usually permanent, meaning no further therapy nor further expense is required. AND, the results of migraine surgery exceed the expected results with Aimovig.
Migraine surgery, however, does not have a $120,000,000,000 company to push the word out about it’s success rates. We as migraine surgeons work very hard individually (an example is this blog) to make migraine patients aware that migraine surgery is an option with outstanding results that exceed those expected with Aimovig or any drug regimen.
Again here I think it is important for me to reiterate that I am in no way against Aimovig or any drug regimen that can treat migraine pain. No matter if it’s a daith piercing, acupuncture, Immitrex, Aimovig, or asparagus juice for that matter, if it works, do it! I’m in favor of any and all means except for narcotics in the effort to alleviate migraine pain. For narcotics, since there is soooo much morbidity that comes from their use, I STRONGLY suggest that patients look into migraine surgery before starting on narcotics. But I’m in favor of patients trying Aimovig or other regimens if they like to see if they can find relief from migraine pain. But, if the cost, side-effects, or efficacy of these other treatments is inadequate in controlling your migraine pain, please see a migraine surgeon for an evaluation. Odds are that we can help, and that migraine surgery can do so better than Aimovig, and for significantly less money than that drug over time.
REFERENCES
1) FDA (2018). FDA approves novel preventive treatment for migraine. [online] Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm608120.htm [Accessed 17 May 2018].
2) Guyuron, B., Reed, D., Kriegler, J. S., Davis, J., Pashmini, N., Amini, S. A placebo-controlled surgical trial of the treatment of migraine headaches. Plast Reconstr Surg 2009;124:461-468.
3) Guyuron, B., Kriegler, J. S., Davis, J., Amini, S. B. Five-year outcome of surgical treatment of migraine headaches. Plast Reconstr Surg 2011;127:603-608.